‘Toy Block’ Synthesis to Revolutionise Polymer Industry
From lab to line: nanotechnology that’s ready to scale.

Imagine snapping nanoparticles onto polymer microparticles like toy blocks—but on a nanometre scale. No messy solvents, no multi-step chemistry—just dry, mechanical collisions that bind materials together.
Doing so would enable manufacturers to better insulate electrical equipment, improve packaging designs for sensitive products, create better catalysts and pharmaceutical delivery systems, improve battery performance, and enhance the wide polymer sector with a range of easily applied properties.
This discovery has now been made at the Korea Electrotechnology Research Institute (KERI) with a breakthrough which could provide polymer manufacturers with all these advantages in a simple toy-like process.

Traditionally, attaching nanoparticles to polymer surfaces has meant using messy, wet, multi-step processes, often involving harsh chemicals and solvents with high environmental and production costs. However, a nanotechnology team led by Dr. Seunggun Yu has been inspired by crater-formation mechanics—like meteorites striking the moon—to create a novel way to attach them.
As the research journal Phys.org explains, “In this process, inorganic nanoparticles are individually attached to the surface of polymer microparticles, forming a core-shell structure where the nanoparticles envelop the polymer microparticles like a shell.”
By carefully tuning particle sizes, collision speeds, surface energies, and roughness, the researchers have optimized nanoparticle coverage and bonding—an impressive engineering feat. In fact, the process is so simple, it should be quickly adoptable by industry.
“Since we can easily combine the materials we need like toy blocks in an eco‑friendly dry process that uses no solvents, it is advantageous for mass production and commercialization,” explains Dr. Seunggun Yu.
Termed ‘mechanophysical synthesis,’ the process involves colliding inorganic nanoparticles with polymer microparticles under controlled speed, energy, and surface conditions. The result is a hybrid core‑shell supraparticle, where nanoparticles coat polymer cores in a single step.
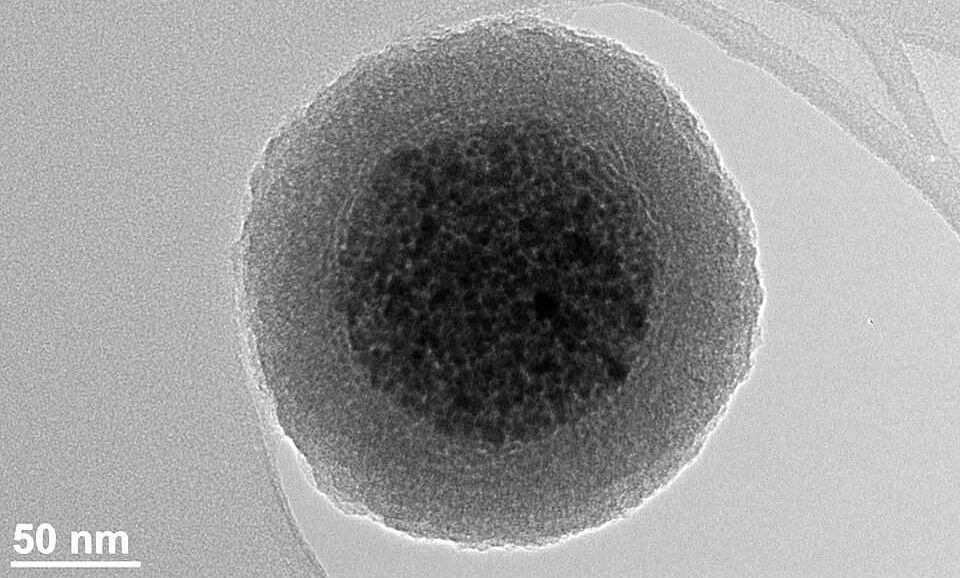
While performing these actions at a scale less than 1/10,000th the width of a human hair may sound complex, the process is inherently scalable. Its simplicity means that existing equipment—rotors, mills, drum mixers—can be adapted to include these hybrid particles, reducing both capital and operational costs.
“This technology has a very wide range of attachable materials,” adds Yu. “And the reproducibility in a simple process is high, which means the entry barrier for industry is very low.”
Extensive testing has shown that these nanoparticles offer thermal, mechanical, and chemical durability, as well as integrated magnetic, photocatalytic, and adsorption properties—a highly desirable set of features for advanced materials.
According to Phys.org, the team is moving swiftly to gain business from the discovery, stating that, “KERI aims to accelerate the optimization of synthesis processes through continuous research. In addition, it plans to actively pursue commercialization by identifying potential industry partners … and promoting technology transfer.”
The team is already reportedly working on scale-up trials and pilot programs—especially in electronics and battery manufacturing—signalling that that market-ready applications may be just around the corner.

Looking ahead, the team foresees the technology being used to create new hybrid combinations of nanoparticles tailored for electrical, magnetic, or optical properties. The process is also compatible with additive manufacturing, offering the potential for 3D-printed composite materials with built-in multifunctionality. Additionally, by attaching dielectric or conductive nanoparticles to polymer beads, manufacturers could develop next-generation insulation, EMI shielding, and heat-dissipating materials for electronic devices—all without relying on toxic solvents.
The method could also be adapted for new coating types that incorporate organic nanoparticles, biomaterials, or even multi-shell architectures—each offering additional performance characteristics and unique selling points. Or alternatively, in high-voltage applications, these durable, multifunctional particles could replace traditional oil-based insulators with lighter, greener alternatives.
All this while remaining an affordable and environmentally sound application of nanotechnology.
Related articles: 5 Polymers that Will Change the World or The 101 on Nanoclay-Enhanced Polymers
Mechanophysical synthesis isn’t just a clever trick—it’s a manufacturing revolution. By treating nanoparticles and polymers like interlocking blocks, nanomaterial specialists have designed a process that’s green, scalable, cost-effective—and ready for industrial use.
From batteries to biomedical carriers, and from smart packaging to electrical insulators, this template technology sets the stage for a new era of enhanced, eco-conscious, multifunctional polymer materials. Or, as Dr. Yu puts it, it’s the ideal combination of “mass production and commercialization” with nanotechnology—finally approachable and implementable for modern manufacturing.
Photo credit: kjpargeter on Freepik, usertrmk, Freepik, & Wikimedia

